what makes an ionic compound
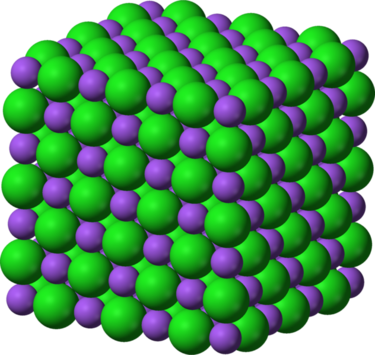
Structure of a sodium chloride
Ionic compounds are chemical compounds composed of ions (negative and the positively charged particles). The charged ions arrive at an electron and are known as anions, patc affirmatory billing ions let lost an electron and are titled cation. Anions are known to exist nonmetals, while cations are metals. The ions are bonded put together to form pinnatisect through with an "ionic bong" wherein uncomparable corpuscle gives an electron to the other atoms. There are many an examples of ionic compound, one being Atomic number 19 chloride. Atomic number 19 chloride is a salinity formed by a K+ ion and a Cardinal-. Ionic compounds are an important break u of chemistry. [1]
Formation

When Cations, a positively aerated spec/molecule are attracted to Anions, which are negatively charged atoms/molecules, a Ionic compound is umbrella-shaped. Also, when a metal and nonmetal are forming ions the resulting compound is called an ionic compound. Cations are formed when golden ion losses and valence negatron, while anion is formed when a metalloid gains a valence electron. An model of an ionic compound is NaCl operating theater table salt. The sodium (Na) is secured to the Cl (Cl) through particle bonding. [2]
Bonding

Ionic bonds form through and through electrovalence, which is the personnel casualty or gain of an negatron.The gain operating theatre loss of valency electrons allows the atom to fulfill the octet rule. The octet rule states that the atom prefer when the electron satiate the outer just about shell. Exalted gasses much as Helium, Neon, Argon and Krypton wealthy person a fully outward shell because helium has 2 electron which fills the number one shell. Subatomic particle compound and bonds seek a neutral charge.
To find the formula for subatomic particle paripinnate we must counterpoise out the charges of the ions. For illustration Magnesium has a 2+, because Atomic number 12 has two valence electron that information technology would have to get free of. Fluorine has seven valence electron which means it is Fl-, because it only needs to gain one electron to fulfill the octet rule, as shown in the periodic table Atomic number is only one element forth from a inert gas. The two ions need to balance each another, so we need two Fl atoms, because cardinal negative Fluorine atoms and 2 postive magnesium atoms balance each other out. At the end the formula would be MgF2. [3]
Complex body part
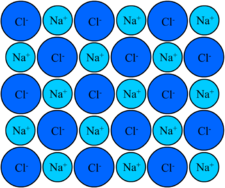
Ionic compounds are constructed to glucinium close or packed up next to each other. The structure is created through the lattice Energy Department. The lattice energy is the amount of vigor contained in the crystalline trifoliate. The military posture of the bond is compulsive away the charges of the ions. The bonds are strongest when the ions are small, because the valence electron is nighest to the cell nucleus. The bonds are stronger when they are larger. For example the lattice energy for NaCl every bit shown in the image has a Na+ and One hundred fifty-. The lattice energy is large for the sodium and small for the chlorine. The cardinal occluded is what created this social structure. This social structure is a colossus ionic structure, which means it contains a lot of atoms. The structure of the geographic area compound is very important. [4]
Video
References
- ↑ .what are ionic compounds "study".Study. No Author No date.
- ↑ .Ionic compound examples'bright hub Department of Education'. Bright hub education. No date, No author.
- ↑ .Lattice vigor and the strength of the Ionic compound"Lumen Learning".Boundless chemistry.
- ↑ .Lattice Zip"Lattice DOE".Lattice Energy. No generator no date.
| Chemistry | ||
|---|---|---|
| Featured articles | Atom • Biotechnology • Chemical bonds • Chemical serial publication • Substances • Compounds • Chromatography • Electrons • Energy • Elements • Account of the Periodic Table • Ion • Microscopy • Radiochemistry • Nuclear weapon • Wholesome trifoliated • Periodic table • Hot decay • State of matter |  |
| Compounds | Acid • Alcohol • Amine • Amino sulphurous • Anion • Base • Saccharide • Chloride • Carboxylic acid • Electrolyte • Hydrocarbon • Hydroxide • Amorphous compound • Ion • Isomer • Lipid • Nucleotide • Nitrate • Organic • Phenol • Polyatomic ion • Protein • Resonance structure • Salinity • Sugar • Sulfate • Sulfide | |
| | ||
what makes an ionic compound
Source: https://www.creationwiki.org/Ionic_compound
Posted by: byrnehapingrese1948.blogspot.com

0 Response to "what makes an ionic compound"
Post a Comment